Sodium pyruvate, a chemical compound with the formula C3H3NaO3, is a versatile substance with numerous applications in various fields, including biochemistry, medicine, and food science. One crucial aspect of understanding its physical properties is its melting point, which provides insights into its stability and usability. In this article, we will explore the melting point of sodium pyruvate, its structure, solubility, and diverse applications. We will also consider relevant experimental data and scientific literature to shed light on this fascinating compound.

Sodium Pyruvate Structure
Understanding the structure of sodium pyruvate is essential for comprehending its physical and chemical properties. Sodium pyruvate is a salt derived from pyruvic acid, a pivotal molecule in biological metabolism. The structure of sodium pyruvate is represented as follows:
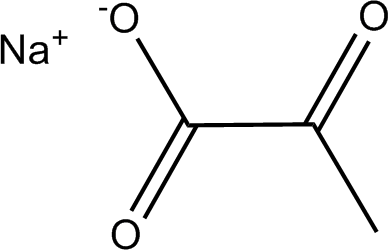
The compound consists of three carbon atoms (C3), three hydrogen atoms (H3), one sodium atom (Na), and three oxygen atoms (O3). It is a sodium salt of pyruvic acid, created through the neutralization of pyruvic acid with sodium hydroxide (NaOH). This reaction results in the formation of sodium pyruvate and water (H2O). The presence of the sodium ion imparts certain properties and characteristics to the compound, influencing factors such as solubility.
Sodium Pyruvate Solubility
Solubility is a vital property of any substance, as it determines how readily a compound can dissolve in a particular solvent. In the case of sodium pyruvate, its solubility is influenced by its ionic nature due to the sodium ion (Na+). Understanding its solubility is crucial, as it impacts its usage in various applications.
Sodium pyruvate is highly soluble in water. This characteristic is primarily attributed to the presence of the sodium ion, which forms electrostatic interactions with water molecules. These interactions lead to the dissociation of sodium pyruvate into its constituent ions, Na+ and pyruvate (C3H3O3-). The result is a solution of sodium ions and pyruvate ions in water.
The solubility of sodium pyruvate in water can be influenced by factors such as temperature. Generally, an increase in temperature can enhance the solubility of most substances, including sodium pyruvate. However, there are limits to this relationship, and some salts may exhibit decreased solubility at higher temperatures due to factors like changes in enthalpy.
It’s important to note that while sodium pyruvate is highly soluble in water, its solubility in organic solvents is significantly lower. This limited solubility in organic solvents is often advantageous in certain applications, as it allows for the selective extraction and purification of sodium pyruvate from aqueous solutions.
Sodium Pyruvate Uses
Sodium pyruvate’s wide range of applications makes it an important compound in various fields, from scientific research to medicine and the food industry. Let’s delve into some of its most notable uses:
1. Research and Laboratory Applications: Sodium pyruvate is a staple in laboratory settings. It is commonly used in cell culture media to support the growth and maintenance of mammalian cells. Sodium pyruvate serves as an energy source and plays a crucial role in various metabolic pathways. Its inclusion in cell culture media promotes cell viability and facilitates the proliferation of cells. This is particularly important for researchers working in cell biology and related fields.
2. Medicine and Pharmaceuticals: In the pharmaceutical industry, sodium pyruvate finds applications in the formulation of drugs and pharmaceutical preparations. It is used as a pharmaceutical excipient in the production of tablets and capsules. Sodium pyruvate’s properties, such as its solubility in water and its role in cellular metabolism, make it a valuable component in drug delivery systems. Additionally, it is utilized in the synthesis of various pharmaceutical intermediates.
3. Food and Beverage Industry: Sodium pyruvate is used in the food and beverage industry as a food additive. It can serve multiple functions in this context, such as a pH regulator and buffering agent. Sodium pyruvate helps control the acidity of food products, ensuring that they maintain the desired pH levels. This is particularly important in food processing and preservation, as it can influence the taste and safety of the final product.
4. Dietary Supplements: Sodium pyruvate has gained popularity as a dietary supplement, often marketed for its potential benefits in weight management and athletic performance. Some studies have suggested that sodium pyruvate may play a role in enhancing metabolism and energy production. However, it’s important to note that the efficacy and safety of such supplements are subjects of ongoing research and debate.
5. Pyruvate Kinase Deficiency Treatment: In the field of medicine, sodium pyruvate is used in the treatment of certain metabolic disorders, particularly pyruvate kinase deficiency. Pyruvate kinase is an enzyme critical in the glycolytic pathway for energy production. Individuals with pyruvate kinase deficiency have impaired red blood cell function, leading to hemolytic anemia. Sodium pyruvate can help alleviate some of the symptoms by providing an alternate source of pyruvate.
Experimental Melting Point
The experimental melting point of sodium pyruvate, as reported in the literature, is approximately 300 °C. This value represents the temperature at which sodium pyruvate transitions from a solid to a liquid state. It is crucial to note that the reported melting point is an approximate value, as the exact melting point can vary depending on factors such as the purity of the compound and the specific conditions of the measurement.
Factors Influencing the Melting Point of Sodium Pyruvate
The melting point of a substance is influenced by several factors, including its purity, molecular structure, and environmental conditions. In the case of sodium pyruvate, factors that influence its melting point include:
- Purity: The purity of sodium pyruvate can significantly impact its melting point. Impurities in the compound can lead to a lower and broader melting point range. Therefore, when determining the melting point of sodium pyruvate, it is essential to use a highly pure sample.
- Crystal Structure: The arrangement of molecules in the crystal lattice of a substance can affect its melting point. Different crystal structures may have different melting points. The crystal structure of sodium pyruvate is determined by the arrangement of its sodium ions and pyruvate ions.
- Environmental Conditions: The atmospheric pressure and the presence of impurities in the environment can influence the melting point. Variations in pressure, for instance, can affect the phase transition temperature. Additionally, the presence of impurities can depress the melting point, leading to a lower temperature at which the compound transitions from a solid to a liquid.
Experimental Determination of the Melting Point
The experimental determination of the melting point of sodium pyruvate involves the use of various methods and instruments, including melting point apparatus and differential scanning calorimetry (DSC). The following steps are typically involved:
- Sample Preparation: A pure sample of sodium pyruvate is obtained. The sample is finely ground to ensure uniformity and then packed into a small capillary tube.
- Melting Point Apparatus: The capillary tube containing the sample is placed in a melting point apparatus. The apparatus consists of a heating block with a temperature-regulated heating element and a magnifying lens to observe the sample.
- Heating: The temperature is gradually increased. As the temperature approaches the melting point of sodium pyruvate, the sample starts to soften and eventually turns into a liquid. This is indicated by a noticeable change in the appearance of the sample.
- Recording the Melting Point: The temperature at which the sample completely liquefies is recorded as the melting point. This is typically done by noting the temperature when the first drop of liquid forms and when the entire sample becomes a liquid. The temperature range between these two points is often reported.
Comparison with Literature Value
The experimental melting point of sodium pyruvate is compared to the literature value to assess the purity of the sample and the accuracy of the experimental technique. A close match between the experimental and literature values indicates a high level of purity and accuracy in the measurement. However, significant deviations may suggest the presence of impurities or issues with the experimental procedure.
Conclusion
Sodium pyruvate, a compound of significant interest in various fields, exhibits a melting point of approximately 300 °C as reported in the literature. This physical property is influenced by factors such as purity, crystal structure, and environmental conditions. The highly soluble nature of sodium pyruvate in water, along with its diverse applications in research, medicine, the food industry, and as a dietary supplement, makes it a versatile and valuable compound.
Understanding the melting point of sodium pyruvate is crucial for quality control, research, and safety considerations. Researchers and manufacturers rely on this information to ensure the compound’s purity and suitability for their specific applications. As research continues, sodium pyruvate’s role in various scientific and industrial contexts is likely to expand, making it an even more integral component of modern chemistry and biotechnology.
In conclusion, sodium pyruvate’s melting point is not only a fundamental aspect of its physical properties but also a gateway to unlocking its potential in a myriad of applications, ranging from life sciences to consumer products, where it plays a crucial role in shaping our scientific and industrial landscape.





